EnzLab SE (Security Edition)
EnzLab SE (Security Edition) is a parallel version of the standard EnzLab software, which fulfills the specific FDA requirements of 21 CFR Part 11. These are requirements in terms of user administration, data security and documentation of results and actions. Except from that, EnzLab SE is basically compatible to the EnzLab standard version, but this variant remains at the level of Enzlab 4.0.
EnzLab SE utilizes the Windows user administration: Users are configured and administered by means of operating system functions. This basically is done in the Installation Qualification procedure. For EnzLab SE users can be defined on the normal level (Operator) and on an expert level (Supervisor). Only a user on the supervisor level has access to method definition and system configuration.
Methods and results files are encoded and protected by a checksum. Thus the files cannot be modified and can only by viewed within the software. The system is configured such that he method and results directories cannot be deleted or moved. When created or modified, method and result files are commeneted and signed electronically by the user. This information is recorded in the audit trail section of the files.
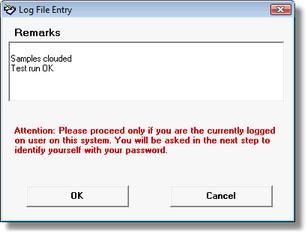
Figure 1 EnzLab SE Signature Window
EnzLab SE administers an encoded logbook file. All relevant actions are automatically registered. Also the user may insert individual entries and sign with his electron signature.
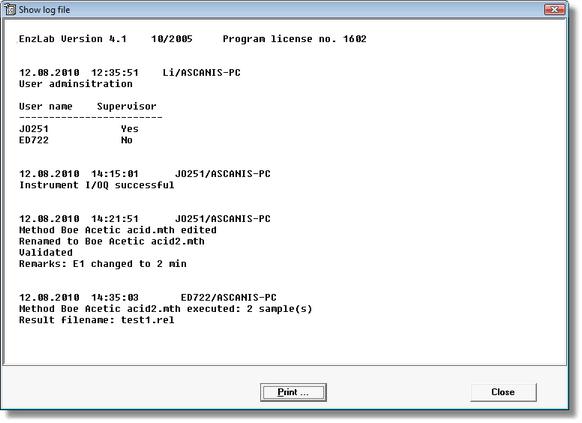
Figure 2 EnzLab SE Log File
With these features, EnzLab SE is designed for use in pharmaceutical applications.
Requirements: PC with Windows XP Professional or 7 Professional.